Features
-
COMMUNICATION.
The VeriDoc 2C systems have a USB port, via which the process data for electronic documentation and tracking purposes or for statistical analysis can be transferred to a USB stick or external hard drive.
-
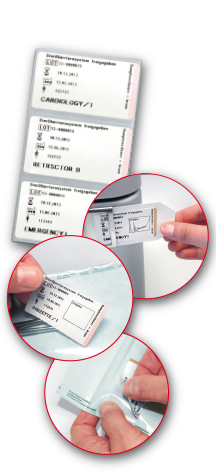
ValiPrint – LABEL PRINTER.
Together with the practical indicator labels, it also provides helpful instrument labelling that complies with legal requirements. The following labels are available:
- Steam (STEAM)
- Steam / Ethylene oxid (STEAM/ETO) combination label
- Steam / Formaldehyde (STEAM/FORM) combination label
- Plasma (VH2O2)
A second ValiPrint label printer can be optionally connected to the new VeriDoc 2C. This means that 2 printers with 2 different labels (e.g. STEAM and VH202) can be operated with one system. -
INTEGRATION.
Regardless of whether it’s sealable pouches and reels, wrapped sets or trays or reusable containers, VeriDoc 2C assists medical professionals to meet packaging labelling requirements. It also includes sterilization inspection using a process indicator along with formal storage approval after a visual inspection, as well as documentation in the patient’s file.
After the required visual inspection of the packaging, the system is informed whether or not it has passed by scanning the corresponding Data Matrix code (‘sterile barrier system approved’ or ‘sterile barrier system not approved’). Depending on the result of the visual inspection, a label is printed with the corresponding note. The packaging may not be used if an error (e.g. open or broken seal or closure system) is detected.
All required information such as batch number, name of the packer, product information or the expiry date is simply transferred to the labels via the easy-to-use ValiScan 2D barcode scanner and an individualized Data Matrix code list. After the sterilization, the integrated process indicator (ISO 11140-1, Class 1) shows whether or not the packaged instrument has been sterilized. After sterilization, personalized approval can be given for storage using the field provided. After the instrument has been stored and used, the so- called duplex-label can be removed from the sterile barrier system and placed in an appendix to the patient’s file. This enables the user to immediately determine that each instrument, set or container being used has been properly packaged, sterilized and approved for storage. Instead of the approval field, all data can also be displayed as QR code. The data on the label can be easily transferred to electronic documentation systems or patient‘s file in this way