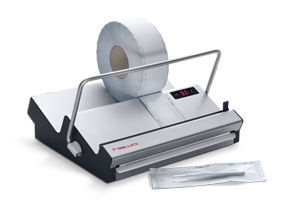
Safe packaging.
The correct reprocessing process of medical devices consists of the steps of washing & disinfection, packaging and sterilisation. The instruments can only be called sterilised when they are packaged before the sterilisation. The single-use (!) packaging, made from laminated poly film and a porous material (Tyvek® or medical grade paper) is permeable for the sterilisation medium (e.g. steam, plasma, Formaldehyde FO or Ethylenoxide ETO), but not for bacteria or microorganisms. Only by following this reprocessing sequence and by using professional heat sealers to seal the instruments as well as professional packaging material can the sterility up to the point of use as well as the aseptic presentation of the instrument be guaranteed.
Medical devices delivered in a sterile state should be packed to ensure that they remain sterile until the point of use. The validation of packaging processes is crucial to ensure that sterile barrier system integrity is attained and will remain so until opened by the users.
The international packaging standard ISO 11607-2 as well as the Technical Specification draft ISO/DTS 16775 explain how packaging processes should be validated. The international packaging validation guideline gives guidance how to validate packaging processes. During the validation of the heat sealing process the optimum sealing temperature of the packaging materials used has to be evaluated and established. At this temperature, the process then has to deliver optimum seal seams that are strong enough and peelable.
Professional heat sealing equipment for closing sealable pouches and reels (preformed sterile barrier systems) are essential for this. hawo therefore offers sealing devices whose processes can be validated according to ISO 11607-2, the Technical Specification draft ISO/DTS 167751 and in harmony with the international packaging validation guideline. The models marked with ‘V’ feature an integrated function for regulating and monitoring process parameters – the ‘V’ is the key! Sealing devices from hawo therefore guarantee efficient and reproducible packing, even for large volumes of instruments.
Definition:
- IQ Installation Qualification:essential information is checked according to a checklist (e.g. the sealing device is compliant to ISO 11607-2)
- OQ Operational Qualification: the optimal sealing temperature has to be established
- PQ Performance Qualification: determination of the seal strength at the optimal sealing temperature determined in the OQ (after sterilisation)