Features
-
White or Stainless Steal?
Is the classic, less expensive steel housing with white surface adequate for your application? Or do you have very high standards regarding an even more convenient and hygienic cleaning? Simply choose between the standard white or the new stainless steel housing (S).

-
Focus sustainability?
Are sustainable products and processes a key priority for you? For us too! This is why we have consistently focused on resource-saving design and engineering of our products. hawo sealing devices use a fraction of the energy of comparable devices due to innovative sealing technology and features – without any compromise in quality. EcoPak 02 – 07 are marked with our GreenTek label as particularly sustainable devices.

-
Stand-by/Automatic start?
Sustainable or efficient sealing? Both, of course! All EcoPak versions are characterised by a sustainable (only 160 Watts) and efficient (10 m/min) sealing process. You would still like to optimise that? Opt for a version with automatic start and/or stand-by mode.
-
Validatable process?
You require a validatable packaging process? No problem: EcoPak 03, 05, 06 and 07 regulate and monitor all process variables according to ISO 11607-2. If not, all other versions are the perfect economical choice for closing sealable pouches and reels in a professional, fast and secure way.

-
Computer interface?
You need a complete documentation of your sealing process? The EcoPak versions with an integrated RS 232 computer interface allow you to transfer the data output of the sealing device to an external software/path via data cable. The new models EcoPak 06 and 07 feature an impressive range of interfaces: 2 x RS232, 2 x USB A, 1 x Ethernet, and Wi-Fi. For a perfect integration into your workflows.

-
Integrated printer?
Are you tired of using labels to mark your sterile barrier systems? Then directly opt for an EcoPak Version with an integrated printer. Data such as name/ID of wrapper, expiry/sterilisation date, LOT number, etc. can directly be printed on the porous part of the material (outside the sealing seam).

-
IntelligentScan?
You want to avoid manual entry of your relevant device configurations? Our IntelligentScan ready EcoPak versions can help. Optimise your new device with an optional hm 980 barcode scanner and use the included software to create individual scan lists which contain configurations such as temperature settings. Entering data has never been this convenient and secure.
-
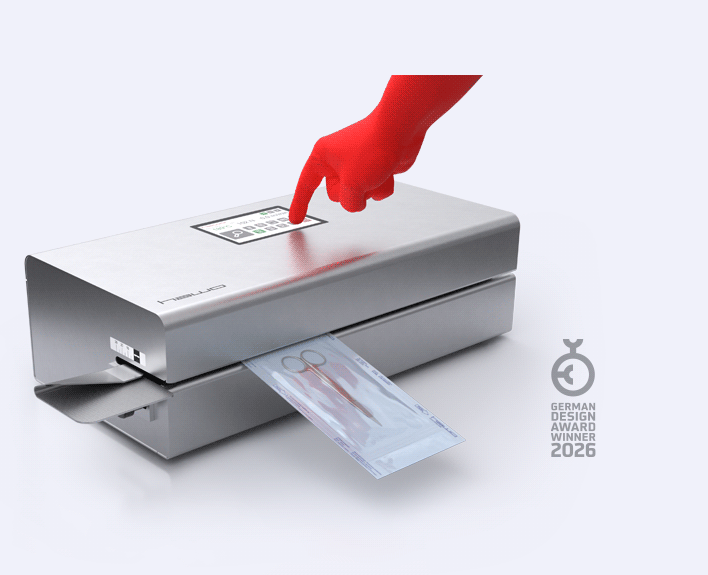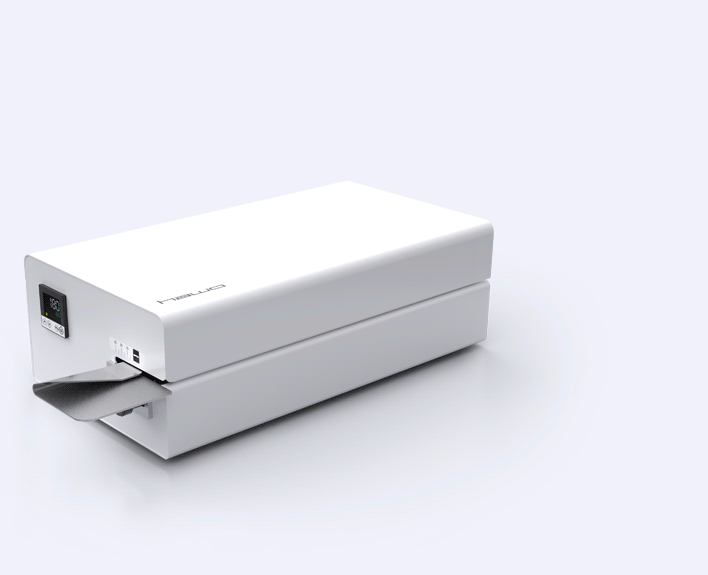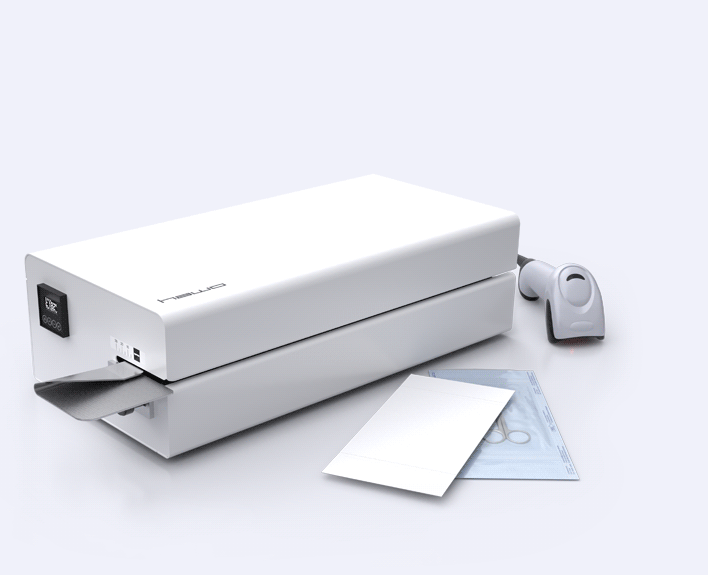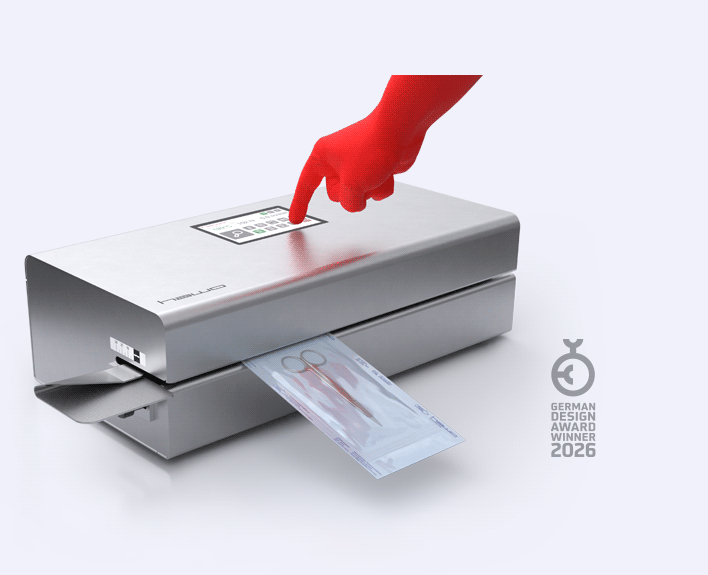
Easy to use?
Our EcoPaks 01–05 are equipped with a modern and clear LCD display that makes them easy to operate. Within the EcoPak series EcoPak 06 and EcoPak 07 offer intuitive touchscreen operation for the first time, making configuration even more user-friendly.